
Major News! JACC Journal: Bioheart Bioabsorbable Scaffold 3-year Follow up Results Released
2025-01-27 13:20:34
Bioabsorbable scaffolds are a new generation of technological breakthrough in interventional therapy forthecoronary heart disease. Compared with traditional permanent stents, bioabsorbable scaffolds have more advantages. After the stent is absorbed, it is beneficial for the positive remodeling of blood vessels, ultimately restoring normal vasoconstriction. Therefore,bioabsorbable scaffolds are hailed as the “fourth revolution in percutaneous coronary intervention”.
The first generation of bioabsorbable scaffolds increased the risk of stent thrombosis and adverse events, which may be related to their relatively thick stent wallsand implantation techniques. The Bioheart bioabsorbable scaffolds area new generation of fully absorbable stents with thinner stent walls. The BIOHEART-II research evaluated theirefficacy and safety.
Recently, the JACC journal released the 3-year follow-up results of the BIOHEART-II research, with Professor Qiao Shubin from Fuwai Hospital, CAMS & PUMC as the corresponding author and Professor Liu Shengwen from Fuwai Hospital, CAMS & PUMC as the first author.

List of clinical trial institutions and researchers participating in BIOHEART II research (in no particular order):
Professor Qiao Shubin, Fuwai Hospital, CAMS & PUMC; Professor Nie Shaoping/Guo Chengjun, Beijing Anzhen Hospital,Capital Medical University; Professor Hou Yuqing, Nanfang Hospital, Southern Medical University; Professor Huang Guangyong, Liaocheng People’s Hospital; Professor Fu Guosheng, SirRun Run Shaw Hospital,Zhejiang University School of Medicine; Professor Zhou Hua, Shanghai EastHospital, Tongji Univesity; Professor Zhang Feng, TEDA International Cardiovascular Hospital; Professor Zhang Chunlai/Lu Feng, Tangshan Workers’ Hospital; Professor Wei Meng, Shanghai Sixth People’s HospitalAffiliated to Shanghai JiaoTong University; Professor Wang Lefeng, Beijing Chao-Yang Hospital,Capital Medical University; Professor Liu Bin, the Second Norman Bethune Hospital of Jilin University; Professor Chen Yuguo, Qilu HospitalofShandong University; Professor Lu Dongfeng/Huang Zheng, the First Affiliated Hospital of Guangzhou Medical University; Professor Gao Yingchun, Inner Mongolia Autonomous Region People’s Hospital; Professor Zhang Jun, Cangzhou Central Hospital; Professor Nie Ruqiong, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University; Professor Xia Yong, the Affiliated Hospital of Xuzhou Medical University; Professor Feng Li, Zhongshan City People’s Hospital; Professor Yang Pingzhen, Zhujiang Hospital of Southern Medical University; Professor Yu Zaixin, Xiangya Hospitalof Central South University; Professor Xu Guangma, the People’s Hospital of Guangxi Zhuang Autonomous Region; Professor Li Xudong, Xuzhou Central Hospital.
Research Design
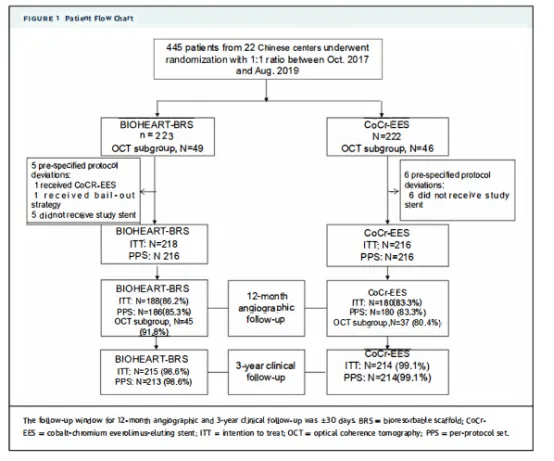
BIOHEART-II,a prospective, multicenter, randomized, non-inferiority trial,enrolled 434 patients in 22 centers in China. The aim was to compare the efficacy of Bioheart bioabsorbable Sirolimus stentsystemand Everolimus eluting coronary stent system in the treatment ofthecoronary heart disease. The primary endpoint was segmental late luminal loss at 12 months, and a 3-year follow-up has been completed.The primary and secondary endpoints were the percentage (%) of stent filament coverage.
Main Research Findings
At the 1-year follow-up, QCA imaging analysis showedno significant difference in late luminal loss within the lesion segment between the Bioheart group and the Everolimus eluting stent group at12 months(0.17 ± 0.38 mm vs. 0.14 ± 0.24 mm, difference of 0.04 mm, 95% CI [-0.02, 0.10 mm]),achievingnon-inferiority(P<0.0001). No differencewas foundin the cumulative percentage of late stage luminal loss between the two groups.
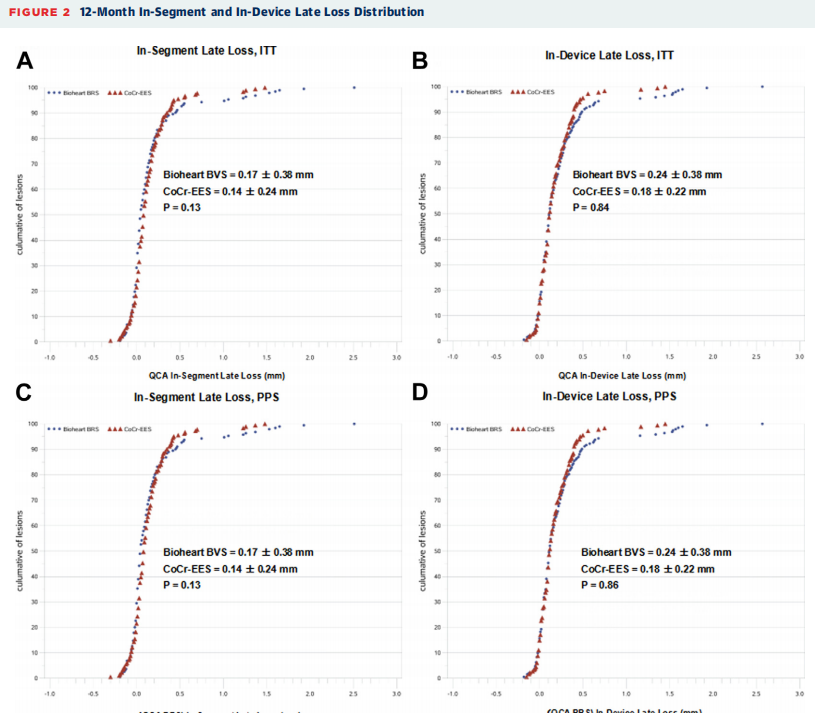
12-Month In-Segment and In-Device LatelossDistributionafter Surgery
The late luminal loss in the Bioheart group was numerically higher than that in the Everolimus eluting stent group (0.24 ± 0.38 mm vs. 0.18 ± 0.22 mm), but the difference was not statistically significant (P=0.84). In addition,there was no significant difference between the two groups in terms of the minimum lumen diameter within the segment, the degree of diameter stenosis within the segment, and the degree of restenosis within the segment.
The analysis of primary and secondary endpoint indicators at the level of stent filaments in the OCT subgroup showed that the percentage of stent filament intimal coverage in the Bioheart group was not inferior to that in the Everolimus eluting stent group at 12 months after surgery (97.9% vs. 98.5%, P<0.0001). In cross-sectional analysis, after surgery and in the1-year follow-up, the incidence of poor stent adhesion in the Bioheart group was lowerthan thatin the Everolimus eluting stent group (P<0.0001).
The 3-year follow-up was conducted on 98.6% and 99.1% of patients in the Bioheart group and Everolimus eluting stent group respectively. In terms of clinical indicators, there was no significant difference in the 3-year target lesion failure rate between the Bioheart group and the Everolimus eluting stent group (5.6% vs. 5.2%, P=0.84).The incidence of patients’related cardiovascular clinical composite endpoints and any single component events was similar between the two groups.
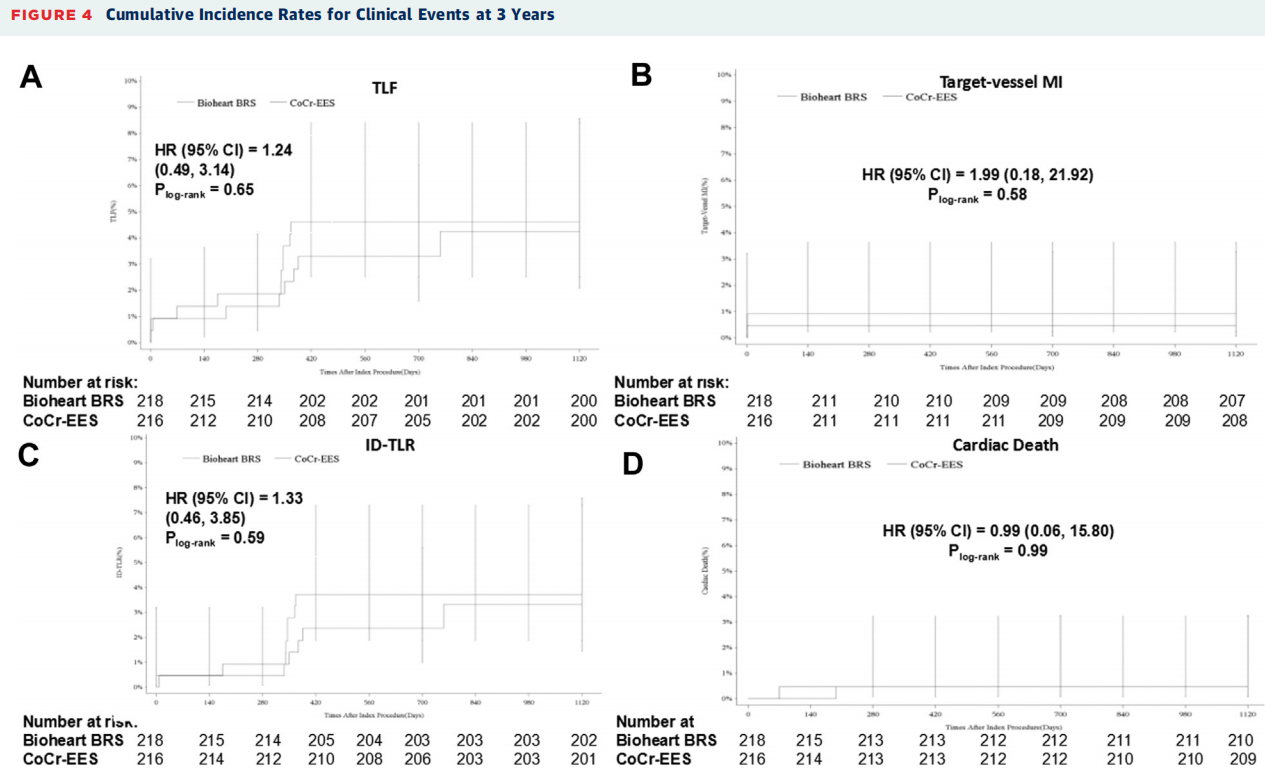
Cumulative Incidence Rates for Clinical Events at 3Years
The incidence of perioperative myocardial infarction was lower in both the Bioheart group and the Everolimus eluting stent group, and the incidence was similar (0.9% vs. 0.5%, P=1.00). During the 3-year follow-up, 1stent thrombosis occurred in the Everolimus eluting stent group, while no stent thrombosis event occurred in the Bioheart group.
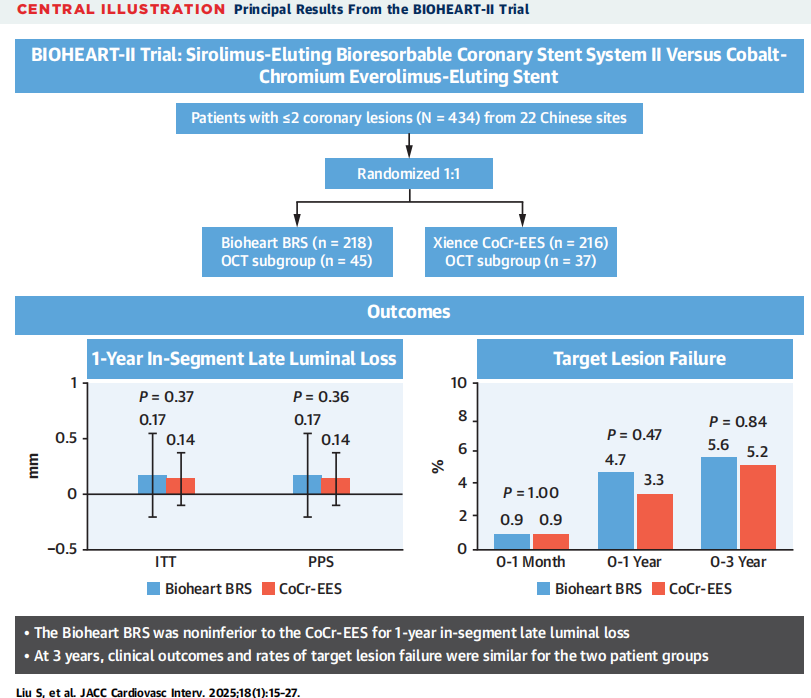
PrincipalResults From theBIOHEART-II Trial
Interpretation of Research Results
The experimental stent used in this research was the new generation Bioheart bioabsorbable coronary artery Sirolimus eluting stent developed by Bioheart. The results were as follows:
①One year after surgery, the segmental luminal loss of Bioheart was not inferior to that of the Everolimus eluting stent; ②At 1 year, the stent filament coveragein Bioheart was not inferior to that of the Everolimus eluting stent; ③In the treatment of non-complex new onset lesions, the incidence of adverse clinical eventsin 3years(including target lesion failure, PoCE, and stent thrombosis) of Bioheart and Everolimus eluting stents is generally low and comparable.
In this research, the results of 1-year average segmental luminal loss (0.17mm) of the Bioheart stent were similar to previous studies, such as the Absorb second-generation bioabsorbable scaffold (0.19mm) in the APPRORB China trial, the NeoVas biodegradable stent trial results from Lepu Medical(0.14mm), and the Firesorb biodegradable stent for minimally invasive medicine (0.17mm)from MicroPort Scientific Corporation. The 1-year segmental luminal loss of the two groups was similar, with luminal loss of Bioheart stentsbeing numerically higher (0.24 mm vs 0.18 mm) compared to that of Everolimus eluting stents. However, this difference did not translate into an increase in clinical restenosis or target lesion revascularization events, which was consistent with previous research findings,indicating that less luminal loss was associated with lower target lesion revascularization rates.
In early Absorb BRS studies, poor stent adhesion was a risk factor for stent thrombosis. In this research, the incidence of poor stent adhesion was lower in the Bioheart group.OCT subgroup studies showed that the stent filament coverage in the Bioheart group was not inferior to that in the control group. The proportion of patients with complete stent filament coverage tended to be higher than that in the control group. The thin wall of the stent may be the main reason for high filament coverage.Good stent adherence promotedendothelialization, which may be an important reason for nooccurrence of stent thrombotic events in the Bioheart group.
About Bioheart Sirolimus-ElutingBioabsorbable Coronary Stent
The Bioheart Sirolimus-eluting bioabsorbable coronary stent is a new generation thin-walled bioabsorbable coronary stent product independently designed and developed by Shanghai Bio-heart Biological Technology Co., Ltd.
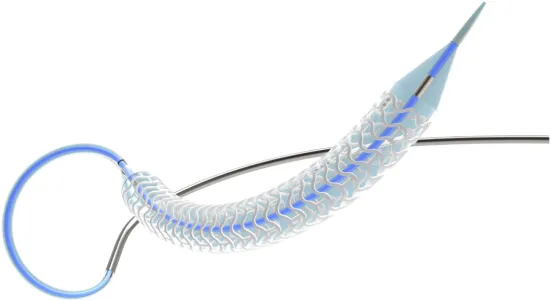
Compared with similar products, this product has the following advantages:
1) The Bioheart stent is composed of in-phase waveform rings connected by direct bridges. By optimizing the structure of the stress concentration area, it can providebetter radial support strength on the premise of thicker walls. The product reduces the wall thickness and shortens the degradation and absorption time as much as possible, effectively reducing the risk of thrombosis.
2) Due to the complete lack of development of the stent material, it is difficult to accurately determine the position of the stent under DSA during and after implantation, making it hardfor doctors to accurately determine whether the lesion area is completely covered and implement effective post expansion. So the development performance of the development point is particularly important on the BRS. The Bioheart stent is designed with a patented development point cake structure and a unique embedding process, ensuring that the developmentmaterial has the maximum area of development while maintaining the firmness and development of the stent embedding, improving the development effect and enhancing the tracking and positioning capacityof the stent during surgery.
3) The Bioheart stent uses a unique spray coating process with patented technology to create a drug coating on the outer surface and sides of the stent embedded in the blood vessel wall. This not only effectively improves the utilization efficiency of drugs, but also enhances the accuracy and stability of drug release.
4) The Bioheart stent adopts theunique “pillow” technology in the clamping process, which forms a head-in-pillow effect at both ends of the stent through the clamping technology with independent intellectual property rights, so as to ensurethat the stent can be stably fixed on the balloon without falling off. In addition, the decrease in profile caused by the thin wall of the stent enables it to have strong passability when passing through complex lesions.